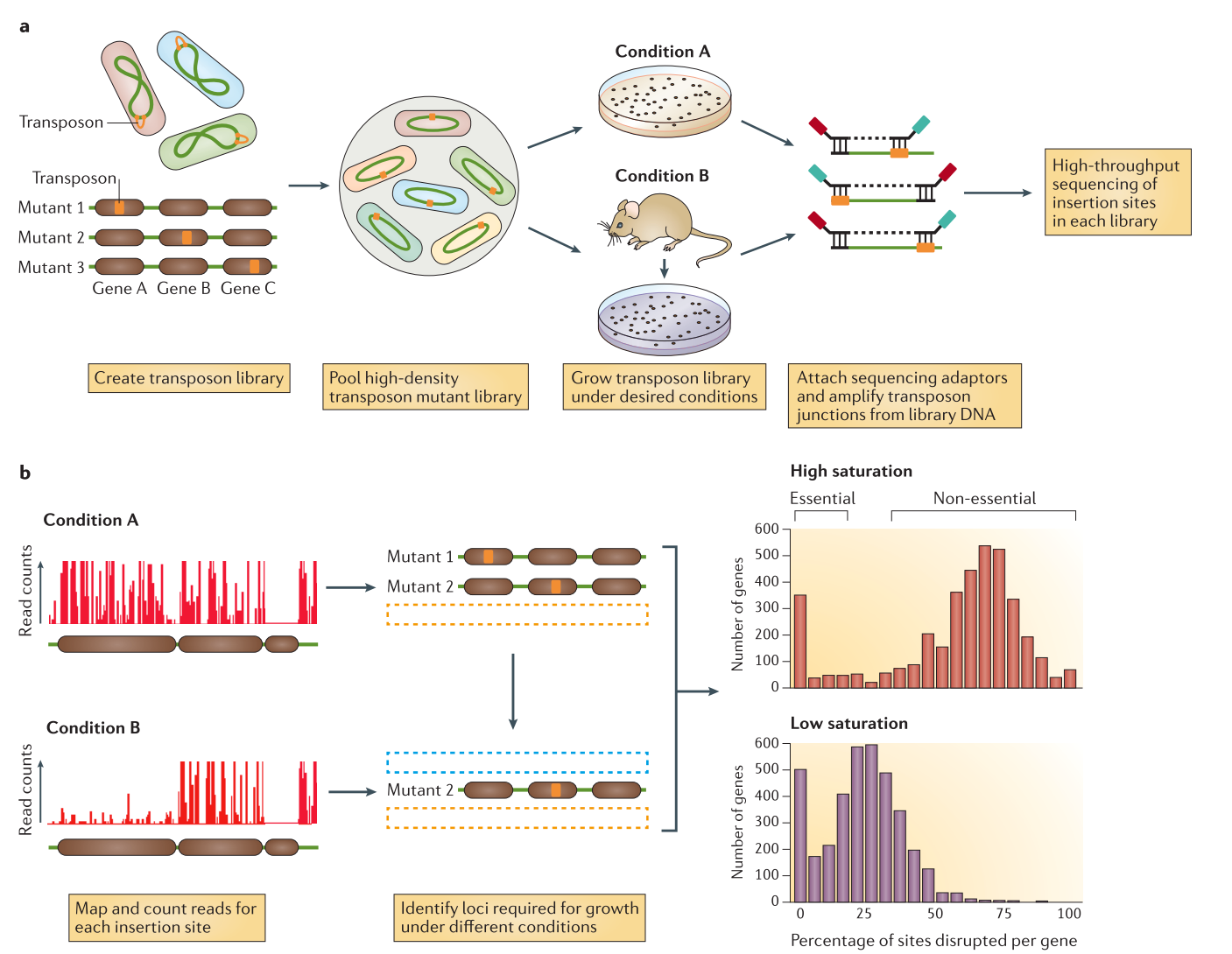
Tn-seq analysis pipeline (improved2)
gene_x 0 like s 742 view s
Tags: pipeline
-
Overview of Data Processing Procedure
1. Convert .fastq files to .fasta format (.reads). "AGCTTCAGGGTTGAGATGTGTATAAGAGACAG", allowed a mismatch of 1 nt 2. Identify reads with the transposon prefix in R1. The sequence searched for is "AGCTTCAGGGTTGAGATGTGTATAAGAGACAG", allowed a mismatch of 1 nt, which must start between cycles 5 and 10 (inclusive). (Note that this ends in the canonical terminus of the Himar1 transposon, TGTTA.) The “staggered” position of this sequence is due to insertion a few nucleotides of variable length in the primers used in the Tn-Seq sample prep protocol (e.g. 4 variants of Sol_AP1_57, etc.). The number of mimatches allowed in searching reads for the transposon sequence pattern can be adjusted as an option in the interface; the default is 1. #What are TACCACGACCA? 3. Extract genomic part of read 1. This is the suffix following the transposon sequence pattern above. However, for reads coming from fragments shorter than the read length, the adapter might appear at the other end of R1, TACCACGACCA. If so, the adapter suffix is stripped off. (These are referred to as “truncated” reads, but they can still be mapped into the genome just fine by BWA.) The length of the genomic part must be at least 20 bp. 3. Extract barcodes from read 2. Read 2 is searched for GATGGCCGGTGGATTTGTGnnnnnnnnnnTGGTCGTGGTAT”. The length of the barcode is typically 10 bp, but can be varaible, and must be between 5-15 bp. 4. Extract genomic portions of read 2. This is the part following TGGTCGTGGTAT…. It is often the whole suffix of the read. However, if the read comes from a short DNA fragment that is shorter than the read length, the adapter on the other end might appear, in which case it is stripped off and the nucleotides in the middle representing the genomic insert, TGGTCGTGGTATxxxxxxxTAACAGGTTGGCTGATAAG. The insert must be at least 20 bp long (inserts shorter than this are discarded, as they might map to spurious locations in the genome). 5. Map genomic parts of R1 and R2 into the genome using BWA. Mismatches are allowed, but indels are ignored. No trimming is performed. BWA is run in ‘sampe’ mode (treating reads as pairs). Both reads of a pair must map (on opposite strands) to be counted. 6. Count the reads mapping to each TA site in the reference genome (or all sites for Tn5). 7. Reduce raw read counts to unique template counts. Group reads by barcode AND mapping location of read 2 (aka fragment “endpoints”). 8. Output template counts at each TA site in a .wig file. 9. Calculate statistics like insertion_density and NZ_mean. Look for the site with the max template count. Look for reads matching the primer or vector sequences. -
quality control
./240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R1_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R2_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R1_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R2_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R1_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R2_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R1_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R2_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R1_001.fastq.gz ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R2_001.fastq.gz #from fastqc results of initial_mutants 49821406 35-161 49821406 35-161 https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1194086 https://www.ncbi.nlm.nih.gov/Traces/wgs/AKKR01? https://www.ncbi.nlm.nih.gov/Traces/wgs/AKKR01?display=download #Found 4,131 proteins -
modify the tpp scripts
vim ~/.local/lib/python3.10/site-packages/pytpp/tpp_tools.py #search for "DEBUG" #-maxreads 10000 or not_given for take all! #-primer AGATGTGTATAAGAGACAG the default primer of Tn5 is TAAGAGACAG! #-primer-start-window 0,159 set 0,159 as default! #delete to import barcode-file, since we already demultipled the file! # pattern for read 2... # TAGTGGATGATGGCCGGTGGATTTGTG GTAATTACCA TGGTCGTGGTAT CCCAGCGCGACTTCTTCGGCGCACACACC TAACAGGTTGGCTGATAAGTCCCCG?AGAT AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGT # -----const1---------------- --barcode- ---const2--- ------genomic---------------- ------const3-------------------------------------------------------------- -
run Transposon Position Profiling (TPP) on multiple contigs
#https://transit.readthedocs.io/en/latest/transit_running.html; #https://orca2.tamu.edu/tom/iLab.html # Break-down of total reads (49821406): # 29481783 reads (59.2%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (20339623): #primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG # primer_matches: 0 reads (0.0%) contain CTAGAGGGCCCAATTCGCCCTATAGTGAGT (Himar1) # vector_matches: 0 reads (0.0%) contain CTAGACCGTCCAGTCTGGCAGGCCGGAAAC (phiMycoMarT7) # adapter_matches: 0 reads (0.0%) contain GATCGGAAGAGCACACGTCTGAACTCCAGTCAC (Illumina/TruSeq index) # misprimed_reads: 0 reads (0.0%) contain Himar1 prefix but don't end in TGTTA #kr11.trimmed1_failed_trim 22072406 #-rw-rw-r-- 1 jhuang jhuang 2,7G Jun 11 15:44 kr11.trimmed1 20339623 #-rw-rw-r-- 1 jhuang jhuang 2,9G Jun 11 15:46 kr11.trimmed2 20339623 #cat initial_mutants_a_2_S6_R1_001.fastq | echo $((`wc -l` / 4)) 49821406=29481783 reads (59.2%) + 20339623 = 49821406 #vs. # Break-down of total reads (49821406): # 29356859 reads (58.9%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (20464547): # primer_matches: 0 reads (0.0%) contain CTAGAGGGCCCAATTCGCCCTATAGTGAGT (Himar1) # vector_matches: 0 reads (0.0%) contain CTAGACCGTCCAGTCTGGCAGGCCGGAAAC (phiMycoMarT7) # adapter_matches: 0 reads (0.0%) contain GATCGGAAGAGCACACGTCTGAACTCCAGTCAC (Illumina/TruSeq index) # misprimed_reads: 0 reads (0.0%) contain Himar1 prefix but don't end in TGTTA # read_length: 100 bp # mean_R1_genomic_length: 73.4 bp # mean_R2_genomic_length: 86.4 bp #conda deactivate ##Test AGCTTCAGGGTTGAGATGTGTATAAGAGACAG --> TAAGAGACAG, the results are similar! # Note that "-primer-start-window 0,161" is invalid and cannot replace the default value! #python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R1_001.fastq.gz -reads2 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R2_001.fastq.gz -output kr11_10nt_primer -primer TAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 #mv tpp.cfg kr11_10nt_primer_tpp.cfg #for initial_mutants python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R1_001.fastq.gz -reads2 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R2_001.fastq.gz -output initial_mutants -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 mv tpp.cfg initial_mutants_tpp.cfg #primer_start_window 0,159 # Break-down of total reads (49821406): # 29481783 reads (59.2%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (20339623): # primer_matches: 0 reads (0.0%) contain CTAGAGGGCCCAATTCGCCCTATAGTGAGT (Himar1) # vector_matches: 0 reads (0.0%) contain CTAGACCGTCCAGTCTGGCAGGCCGGAAAC (phiMycoMarT7) # adapter_matches: 0 reads (0.0%) contain GATCGGAAGAGCACACGTCTGAACTCCAGTCAC (Illumina/TruSeq index) # misprimed_reads: 0 reads (0.0%) contain Himar1 prefix but don't end in TGTTA #for LB_culture python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R2_001.fastq.gz -output LB_culture -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 mv tpp.cfg LB_culture_tpp.cfg #for growthout_control_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R2_001.fastq.gz -output growthout_control_24h -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 mv tpp.cfg growthout_control_24h_tpp.cfg #for extracellular_mutants_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R2_001.fastq.gz -output extracellular_mutants_24h -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 mv tpp.cfg extracellular_mutants_24h_tpp.cfg #for intracellular_mutants_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref WA-314_m_.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R2_001.fastq.gz -output intracellular_mutants_24h -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem -replicon-ids contig_2_10,contig_2_9,contig_2_8,contig_2_7,contig_2_6,contig_2_5,contig_2_3,contig_2_2,contig_5_10,contig_5_11,contig_5_12,contig_5_13,contig_5_15,contig_5_16,contig_5_17,contig_5_18,contig_5_9,contig_5_8,contig_5_7,contig_5_6,contig_5_5,contig_5_4,contig_5_3,contig_5_2,contig_5_1,contig_4_2,contig_4_1,contig_3_59,contig_3_58,contig_3_57,contig_3_56,contig_3_55,contig_3_54,contig_3_53,contig_3_52,contig_3_51,contig_3_50,contig_3_49,contig_3_48,contig_3_47,contig_3_46,contig_3_44,contig_3_43,contig_3_42,contig_3_41,contig_3_40,contig_3_39,contig_3_38,contig_3_37,contig_3_36,contig_3_35,contig_3_34,contig_3_33,contig_3_32,contig_3_31,contig_3_30,contig_3_29,contig_3_28,contig_3_27,contig_3_26,contig_3_25,contig_3_24,contig_3_23,contig_3_22,contig_3_21,contig_3_20,contig_3_17,contig_3_16,contig_3_15,contig_3_14,contig_3_13,contig_3_12,contig_3_11,contig_3_9,contig_3_8,contig_3_7,contig_3_6,contig_3_5,contig_3_3,contig_3_2,contig_3_1,contig_1_48,contig_1_47,contig_1_46,contig_1_45,contig_1_44,contig_1_43,contig_1_42,contig_1_41,contig_1_40,contig_1_39,contig_1_38,contig_1_37,contig_1_34,contig_1_33,contig_1_32,contig_1_31,contig_1_28,contig_1_27,contig_1_26,contig_1_25,contig_1_24,contig_1_22,contig_1_20,contig_1_19,contig_1_18,contig_1_17,contig_1_16,contig_1_15,contig_1_14,contig_1_13,contig_1_12,contig_1_10,contig_1_8,contig_1_7,contig_1_6,contig_1_5,contig_1_4,contig_1_3,contig_1_2,contig_1_1,contig_C8715,contig_C8943,contig_C9371,contig_C8939,contig_C9357,contig_C8991,contig_C9445,contig_C8689 mv tpp.cfg intracellular_mutants_24h_tpp.cfg -
generate statistics tables in Excel-format from the multiple contig running.
for sample in initial_mutants LB_culture growthout_control_24h extracellular_mutants_24h intracellular_mutants_24h; do echo "cd ${sample}" echo "cp ${sample}.tn_stats ${sample}.tn_stats_" echo "#Delete all general statistics before the table data in ${sample}.tn_stats_; delete the content after \"# FR_corr (Fwd templates vs. Rev templates):\"" echo "sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_" echo "sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_" echo "python3 ~/Scripts/parse_tn_stats.py ${sample}.tn_stats_ ${sample}.tn_stats.xlsx" echo "#calculate the sum of the first and second columns by \"=SUM(B2:B130)\" and \"=SUM(C2:C130)\"" echo "mkdir ${sample}_wig" echo "mv *.wig ${sample}_wig/" echo "zip -r ${sample}_wig.zip ${sample}_wig/" done cd initial_mutants cp initial_mutants.tn_stats initial_mutants.tn_stats_ #Delete all general statistics before the table data in initial_mutants.tn_stats_; delete the content after "# FR_corr (Fwd templates vs. Rev templates):" sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_ sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_ python3 ~/Scripts/parse_tn_stats.py initial_mutants.tn_stats_ initial_mutants.tn_stats.xlsx #calculate the sum of the first and second columns by "=SUM(B2:B130)" and "=SUM(C2:C130)" #16,228,513 and 2,454,346 cd LB_culture cp LB_culture.tn_stats LB_culture.tn_stats_ #Delete all general statistics before the table data in LB_culture.tn_stats_; delete the content after "# FR_corr (Fwd templates vs. Rev templates):" sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_ sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_ python3 ~/Scripts/parse_tn_stats.py LB_culture.tn_stats_ LB_culture.tn_stats.xlsx #calculate the sum of the first and second columns by "=SUM(B2:B130)" and "=SUM(C2:C130)" #19735541, 3266320 cd growthout_control_24h cp growthout_control_24h.tn_stats growthout_control_24h.tn_stats_ #Delete all general statistics before the table data in growthout_control_24h.tn_stats_; delete the content after "# FR_corr (Fwd templates vs. Rev templates):" sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_ sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_ python3 ~/Scripts/parse_tn_stats.py growthout_control_24h.tn_stats_ growthout_control_24h.tn_stats.xlsx #calculate the sum of the first and second columns by "=SUM(B2:B130)" and "=SUM(C2:C130)" #23812866, 3487969 cd extracellular_mutants_24h cp extracellular_mutants_24h.tn_stats extracellular_mutants_24h.tn_stats_ #Delete all general statistics before the table data in extracellular_mutants_24h.tn_stats_; delete the content after "# FR_corr (Fwd templates vs. Rev templates):" sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_ sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_ python3 ~/Scripts/parse_tn_stats.py extracellular_mutants_24h.tn_stats_ extracellular_mutants_24h.tn_stats.xlsx #calculate the sum of the first and second columns by "=SUM(B2:B130)" and "=SUM(C2:C130)" #6491071, 236041 cd intracellular_mutants_24h cp intracellular_mutants_24h.tn_stats intracellular_mutants_24h.tn_stats_ #Delete all general statistics before the table data in intracellular_mutants_24h.tn_stats_; delete the content after "# FR_corr (Fwd templates vs. Rev templates):" sed -i 's/read_count (TA sites only, for Himar1)/read_counts/g' *.tn_stats_ sed -i 's/NZ_mean (among templates)/NZ_mean (mean template count over non-zero TA sites)/g' *.tn_stats_ python3 ~/Scripts/parse_tn_stats.py intracellular_mutants_24h.tn_stats_ intracellular_mutants_24h.tn_stats.xlsx #calculate the sum of the first and second columns by "=SUM(B2:B130)" and "=SUM(C2:C130)" #20619934, 1402849 mkdir initial_mutants_wig mv *.wig initial_mutants_wig/ cd initial_mutants_wig/ python3 ~/Scripts/update_wig_initial_mutants.py cd .. zip -r initial_mutants_wig.zip initial_mutants_wig/ mkdir LB_culture_wig mv *.wig LB_culture_wig/ cd LB_culture_wig/ python3 ~/Scripts/update_wig_LB_culture.py cd .. zip -r LB_culture_wig.zip LB_culture_wig/ mkdir growthout_control_24h_wig mv *.wig growthout_control_24h_wig/ cd growthout_control_24h_wig/ python3 ~/Scripts/update_wig_growthout_control_24h.py cd .. zip -r growthout_control_24h_wig.zip growthout_control_24h_wig/ mkdir extracellular_mutants_24h_wig mv *.wig extracellular_mutants_24h_wig/ cd extracellular_mutants_24h_wig/ python3 ~/Scripts/update_wig_extracellular_mutants_24h.py cd .. zip -r extracellular_mutants_24h_wig.zip extracellular_mutants_24h_wig/ mkdir intracellular_mutants_24h_wig mv *.wig intracellular_mutants_24h_wig/ cd intracellular_mutants_24h_wig/ python3 ~/Scripts/update_wig_intracellular_mutants_24h.py cd .. zip -r intracellular_mutants_24h_wig.zip intracellular_mutants_24h_wig/ zip -r genbank_files.zip genbank_files -
run Transposon Position Profiling (TPP) on merged_genome
#for initial_mutants python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R1_001.fastq.gz -reads2 240606_VH00358_96_AAFCFJGM5/kr11/initial_mutants_a_2_S6_R2_001.fastq.gz -output initial_mutants_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem mv tpp.cfg initial_mutants_tpp_run2.cfg #for LB_culture python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr13/LB_culture_a_2_S7_R2_001.fastq.gz -output LB_culture_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem mv tpp.cfg LB_culture_tpp_run2.cfg #for growthout_control_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr15/growthout_control_24h_a_2_S8_R2_001.fastq.gz -output growthout_control_24h_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem mv tpp.cfg growthout_control_24h_tpp_run2.cfg #for extracellular_mutants_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr17/extracellular_mutants_24h_a_2_S9_R2_001.fastq.gz -output extracellular_mutants_24h_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem mv tpp.cfg extracellular_mutants_24h_tpp_run2.cfg #for intracellular_mutants_24h python3 ~/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R2_001.fastq.gz -output intracellular_mutants_24h_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem mv tpp.cfg intracellular_mutants_24h_tpp_run2.cfg # "=SUM(B2:B130)" 20619934; "=SUM(C2:C130)" 1402849 # command: python /home/jhuang/.local/bin/tpp -bwa /usr/bin/bwa -protocol Tn5 -ref merged_genome.fasta -reads1 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R1_001.fastq.gz -reads2 ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R2_001.fastq.gz -output intracellular_mutants_24h_run2 -primer AGCTTCAGGGTTGAGATGTGTATAAGAGACAG -mismatches 1 -bwa-alg mem # transposon type: Tn5 # protocol type: Tn5 # bwa flags: # read1: ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R1_001.fastq # read2: ./240606_VH00358_96_AAFCFJGM5/kr19/intracellular_mutants_24h_a_2_S10_R2_001.fastq # ref_genome: merged_genome.fasta # replicon_ids: # total_reads (or read pairs): 51244639 # truncated_reads 0 (genomic inserts shorter than the read length; ADAP2 appears in read1) # trimmed_reads (reads with valid Tn prefix, and insert size>20bp): 23204461 * # reads1_mapped: 20987078 # reads2_mapped: 20915836 # mapped_reads (both R1 and R2 map into genome, and R2 has a proper barcode): 20627374 * (since barcode is deleted, that means only this filters only the records in which the R2 not containing bacterial genome!) # read_count (TA sites only, for Himar1): 20627374 # template_count: 1405508 # template_ratio (reads per template): 14.68 # TA_sites: 4537463 # TAs_hit: 93859 # density: 0.021 # max_count (among templates): 285 # max_site (coordinate): 31693 # NZ_mean (among templates): 15.0 # FR_corr (Fwd templates vs. Rev templates): 0.003 # BC_corr (reads vs. templates, summed over both strands): 0.919 # Break-down of total reads (51244639): # 28040178 reads (54.7%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (23204461): # primer_matches: 0 reads (0.0%) contain CTAGAGGGCCCAATTCGCCCTATAGTGAGT (Himar1) # vector_matches: 0 reads (0.0%) contain CTAGACCGTCCAGTCTGGCAGGCCGGAAAC (phiMycoMarT7) # adapter_matches: 0 reads (0.0%) contain GATCGGAAGAGCACACGTCTGAACTCCAGTCAC (Illumina/TruSeq index) # misprimed_reads: 0 reads (0.0%) contain Himar1 prefix but don't end in TGTTA # read_length: 130 bp # mean_R1_genomic_length: 75.6 bp # mean_R2_genomic_length: 88.5 bp ./initial_mutants.tn_stats # Break-down of total reads (49821406): # 29481783 reads (59.2%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (20339623): --> #16,228,513 and 2,454,346 ./LB_culture.tn_stats # Break-down of total reads (43486192): # 20855173 reads (48.0%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (22631019): --> #19,735,541 and 3,266,320 ./growthout_control_24h.tn_stats # Break-down of total reads (70663823): # 43886543 reads (62.1%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (26777280): ./extracellular_mutants_24h.tn_stats # Break-down of total reads (47473664): # 38115004 reads (80.3%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (9358660): ./intracellular_mutants_24h.tn_stats # Break-down of total reads (51244639): # 28040178 reads (54.7%) lack the expected Tn prefix # Break-down of trimmed reads with valid Tn prefix (23204461): #grep "AGCTTCAGGGTTGAGATGTGTATAAGAGACAG" intracellular_mutants_24h_a_2_S10_R1_001.fastq | wc -l #28404198 #grep "AGCTTCAGGGTTGAGATGTGTATAAGAGACAG" intracellular_mutants_24h_a_2_S10_R2_001.fastq | wc -l #29 #AGCTTCAGGGTTGAGATGTGTATAAGAGACAG #NOTE_IMPORTANT: explain that some multiple mapped reads have to been deleted for the down-stream analysis! -
Prepare the wig files on merged_genome for transit running
#change all wigs title to WA314 #./initial_mutants_run2.wig #./LB_culture_run2.wig #./growthout_control_24h_run2.wig #./intracellular_mutants_24h_run2.wig #./extracellular_mutants_24h_run2.wig sed -i 's/chrom=merged_genome/chrom=WA314/g' *_run2.wig -
Prepare the sample-metadata file for transit running
#my ID Condition Treatment Filename initial_mutants initial_mutants control initial_mutants_run2.wig LB_culture LB_culture control LB_culture_run2.wig growthout_control_24h growthout_control_24h control growthout_control_24h_run2.wig intracellular_mutants_24h intracellular_mutants_24h treated intracellular_mutants_24h_run2.wig extracellular_mutants_24h extracellular_mutants_24h treated extracellular_mutants_24h_run2.wig #Doc Id Condition Filename glyc1 glycerol /Users/example_data/glycerol_rep1.wig glyc2 glycerol /Users/example_data/glycerol_rep2.wig chol1 cholesterol /Users/example_data/cholesterol_rep1.wig chol2 cholesterol /Users/example_data/cholesterol_rep2.wig chol2 cholesterol /Users/example_data/cholesterol_rep3.wig -
Run Transit on merged_genome
#https://training.galaxyproject.org/training-material/topics/genome-annotation/tutorials/tnseq/tutorial.html#compare-the-essential-genes-between-two-conditions #File --> Export --> combined_wig, or IGV, or Mean_Gene_Counts. # --> Convert --> ...... [1] #View --> Scatter_Plot (only two datasets are allowed) # --> Track_View # --> Quality_Control #Analysis --> Himar1_Methods * gumbel * resampling * hmm * example * binomial * griffin * randproduct * utest * gi * normalize * tnseq_stats * corrplot * heatmap * ttnfitness --> Tn5_Methods * resampling: Resampling test of conditional essentiality between two conditions transit resampling -c combined.wig samples.metadata LB_culture intracellular_mutants_24h merged_genome.prot_table resampling_results_test.txt -s 10000 -n TTR -h -a -l -winz [resampling] site_restricted=False [resampling] Starting resampling Method [resampling] Winsorizing insertion counts [resampling] Getting Data Reading combined wig data... Filtering wigs by conditions... Checking condition: initial_mutants, included_conditions: ['lb_culture', 'intracellular_mutants_24h'] Checking condition: LB_culture, included_conditions: ['lb_culture', 'intracellular_mutants_24h'] Checking condition: growthout_control_24h, included_conditions: ['lb_culture', 'intracellular_mutants_24h'] Checking condition: intracellular_mutants_24h, included_conditions: ['lb_culture', 'intracellular_mutants_24h'] Checking condition: extracellular_mutants_24h, included_conditions: ['lb_culture', 'intracellular_mutants_24h'] ['LB_culture' 'intracellular_mutants_24h'] Creating data_ctrl and data_exp arrays... Shapes of data_ctrl and data_exp: data_ctrl.shape: (1, 4537463) data_exp.shape: (1, 4537463) [resampling] Preprocessing Ctrl data... [resampling] Normalizing using: TTR [resampling] Performing LOESS Correction /home/jhuang/.local/lib/python3.10/site-packages/pytransit/stat_tools.py:453: RuntimeWarning: invalid value encountered in divide normalized_Y[window*i:window*(i+1)] = Y[window*i:window*(i+1)] / (ysmooth[i]/mline) [resampling] Preprocessing Exp data... [resampling] Normalizing using: TTR [resampling] Performing LOESS Correction Creating Genes objects... Running resampling... [resampling] Running Resampling Method... 100.0% [resampling] Performing Benjamini-Hochberg Correction Writing output... [resampling] Number of significant conditionally essential genes (Padj<0.05): 37 [resampling] Time: 748.52s [resampling] Finished resampling Method #Resampling #Console: python3 /home/jhuang/.local/bin/transit resampling -c combined.wig samples.metadata LB_culture intracellular_mutants_24h merged_genome.prot_table resampling_results.txt -s 10000 -n TTR -h -a -l -winz #Parameters: samples=10000, norm=TTR, histograms=True, adaptive=True, excludeZeros=False, pseudocounts=1.0, LOESS=True, trim_Nterm=0.0, trim_Cterm=0.0, site_restricted=False, winsorize=True #Control Data: b'lb_culture' #Experimental Data: b'intracellular_mutants_24h' #Annotation path: b'merged_genome.prot_table' #Number of significant conditionally essential genes (Padj<0.05): 37 * utest: Mann-Whitney U-test of conditional essentiality between two conditions. This is a method for comparing datasets from a TnSeq library evaluated in two different conditions, analogous to resampling. transit utest LB_culture_run2.wig intracellular_mutants_24h_run2.wig merged_genome.prot_table utest_out -n TTR -l [utest] Starting Mann-Whitney U-test Method [utest] Getting Data [utest] Normalizing using: TTR [utest] Performing LOESS Correction /home/jhuang/.local/lib/python3.10/site-packages/pytransit/stat_tools.py:453: RuntimeWarning: invalid value encountered in divide normalized_Y[window*i:window*(i+1)] = Y[window*i:window*(i+1)] / (ysmooth[i]/mline) [utest] Running Mann-Whitney U-test Method... 100.0% [utest] Performing Benjamini-Hochberg Correction [utest] Adding File: utest_out_l [utest] Finished Mann-Whitney U-test Method transit utest LB_culture_run2.wig intracellular_mutants_24h_run2.wig merged_genome.prot_table utest_out_without_l -n TTR [utest] Starting Mann-Whitney U-test Method [utest] Getting Data [utest] Normalizing using: TTR [utest] Running Mann-Whitney U-test Method... 100.0% [utest] Performing Benjamini-Hochberg Correction [utest] Adding File: utest_out [utest] Finished Mann-Whitney U-test Method #utest #Console: python3 /home/jhuang/.local/bin/transit utest LB_culture_run2.wig intracellular_mutants_24h_run2.wig merged_genome.prot_table utest_out -n TTR -l #Control Data: b'LB_culture_run2.wig' #Experimental Data: b'intracellular_mutants_24h_run2.wig' #Annotation path: b'merged_genome.prot_table' #Time: 56.52693510055542 #-l := Perform LOESS Correction; Helps remove possible genomic position bias. Default: Turned Off. * ZINB (command line only, If you want to compare more than two conditions, see ZINB.): The ZINB (Zero-Inflated Negative Binomial) method is used to determine which genes exhibit statistically significant variability across multiple conditions, in either the magnitude of insertion counts or local saturation, agnostically (in any one condition compared to the others). Like ANOVA, the ZINB method takes a combined_wig file (which combines multiple datasets in one file) and a samples_metadata file (which describes which samples/replicates belong to which experimental conditions). transit zinb combined.wig samples.metadata merged_genome.prot_table zinb_out -n TTR --condition Condition --include-conditions LB_culture,intracellular_mutants_24h #grep "not analyzed" zinb_out | wc -l #WARNING: Could run successful, but 4097 records are not analyzed! #TODO: R is called by Transit for certain commands, such as ZINB, corrplot, and heatmap. #install R (tested on v3.5.2) #R packages: MASS, pscl, corrplot, gplots (run install.packages(c("MASS", "pscl", "corrplot", "gplots")) install.packages("remotes") remotes::install_version("MASS", version = "7.3-60") #Python packages (for python3): rpy2 (v>=3.0) (run “pip3 install rpy2” on command line) ** ANOVA (command line only): The Anova (Analysis of variance) method is used to determine which genes exhibit statistically significant variability of insertion counts across multiple conditions. Unlike other methods which take a comma-separated list of wig files as input, the method takes a combined_wig file (which combined multiple datasets in one file) and a samples_metadata file (which describes which samples/replicates belong to which experimental conditions). transit anova combined.wig samples.metadata merged_genome.prot_table anova_out -n TTR --include-conditions LB_culture,intracellular_mutants_24h --ref LB_culture -PC 5 -alpha 1000 -winz [anova] Starting Anova analysis [anova] Getting Data [anova] Normalizing using: TTR [anova] Winsorizing insertion counts [anova] Running Anova /home/jhuang/.local/lib/python3.10/site-packages/scipy/stats/_axis_nan_policy.py:531: ConstantInputWarning: Each of the input arrays is constant; the F statistic is not defined or infinite res = hypotest_fun_out(*samples, **kwds) [anova] Adding File: anova_out. 100.0% [anova] Finished Anova analysis [anova] Time: 105.4s #Console: python3 /home/jhuang/.local/bin/transit anova combined.wig samples.metadata merged_genome.prot_table anova_out -n TTR --include-conditions LB_culture,intracellular_mutants_24h --ref LB_culture -PC 5 -alpha 1000 -winz #parameters: normalization=TTR, trimming=0.0/0.0% (N/C), pseudocounts=5, alpha=1000.0 #--ref <cond> := which condition(s) to use as a reference for calculating LFCs (comma-separated if multiple conditions) transit anova combined.wig samples.metadata merged_genome.prot_table anova_5samples_ref_LB_culture_out -n TTR --include-conditions initial_mutants,LB_culture,growthout_control_24h,intracellular_mutants_24h,extracellular_mutants_24h --ref LB_culture -PC 5 -alpha 1000 -winz #TODO_MERGE: merge combined.wig and combined_normalized.wig to Excel-file as the read_counts based on the gene! #Rv Gene TAs Mean_initial_mutants Mean_LB_culture Mean_growthout_control_24h Mean_intracellular_mutants_24h Mean_extracellular_mutants_24h LFC_initial_mutants LFC_LB_culture LFC_growthout_control_24h LFC_intracellular_mutants_24h LFC_extracellular_mutants_24h Fstat Pval Padj status Orf Gene ID. Name Name of the gene. TAs Number of TA sites in Gene Means… Mean readcounts for each condition LFCs… Log-fold-changes of counts in each condition vs mean across all conditions MSR Mean-squared residual MSE+alpha Mean-squared error, plus moderation value p-value P-value calculated by the Anova test. p-adj Adjusted p-value controlling for the FDR (Benjamini-Hochberg) status Debug information (If any) transit example initial_mutants_run2.wig merged_genome.prot_table initial_mutants_mean_read-counts_per_gene.txt #TODO: MERGE anovo+example together, delete all headers of the results, save as the Excel-file! #Console: python3 /home/jhuang/.local/bin/transit anova combined.wig 5samples.metadata merged_genome.prot_table anova_out -n TTR --include-conditions LB_culture,intracellular_mutants_24h --ref LB_culture -PC 5 -alpha 1000 -winz #parameters: normalization=TTR, trimming=0.0/0.0% (N/C), pseudocounts=5, alpha=1000.0 #Rv Gene TAs Mean_LB_culture Mean_intracellular_mutants_24h LFC_LB_culture LFC_intracellular_mutants_24h MSR MSE+alpha Fstat Pval Padj status YWA314_00005 307 185.55 381.95 0.000 1.022 5434118.726573 4314598.914305 1.259473 0.262191 1.000000 - YWA314_00010 243 44.93 32.98 0.000 -0.395 7853.462976 476245.960914 0.016490 0.897874 1.000000 - YWA314_00015 103 0.00 0.00 0.000 0.000 0.000000 0.000000 -1.000000 1.000000 1.000000 No counts in all conditions YWA314_00020 190 1.67 0.00 0.000 -0.415 263.556577 1131.081049 0.233013 0.629578 1.000000 - # ---- commands for one sample ---- ** normalize: Normalization method: python transit.py norm glycerol_H37Rv_rep1.wig,glycerol_H37Rv_rep2.wig H37Rv.prot_table glycerol_TTR.txt -n TTR - TTR: Trimmed Total Reads (TTR), normalized by the total read-counts (like totreads), but trims top and bottom 5% of read-counts. This is the recommended normalization method for most cases, as it has the benefit of compensating for differences in saturation (which is especially important for resampling). - nzmean: Normalizes datasets to have the same mean over the non-zero sites. - totreads: Normalizes datasets by total read-counts, and scales them to have the same mean over all counts. - zinfnb: Fits a zero-inflated negative binomial model, and then divides read-counts by the mean. The zero-inflated negative binomial model will treat some empty sites as belonging to the “true” negative binomial distribution responsible for read-counts while treating the others as “essential” (and thus not influencing its parameters). - quantile: Normalizes datasets using the quantile normalization method described by Bolstad et al. (2003). In this normalization procedure, datasets are sorted, an empirical distribution is estimated as the mean across the sorted datasets at each site, and then the original (unsorted) datasets are assigned values from the empirical distribution based on their quantiles. This actually doesn’t work well on TnSeq data if a large fraction of TA sites have counts of 0 (ties). - betageom: Normalizes the datasets to fit an “ideal” Geometric distribution with a variable probability parameter p. Specially useful for datasets that contain a large skew. - nonorm: No normalization is performed. ** tnseq_stats: Statistical Metrics for TnSeq datasets #Typically a skew < 50 is desired #total_cts Sum of total read-counts in the sample. Indicates how much sequencing material was obtained. Typically >1M reads is desired for Himar1 datasets. transit tnseq_stats -c combined.wig -o tnseq_stats dataset density mean_ct NZmean NZmedian max_ct total_cts skewness kurtosis pickands_tail_index initial_mutants_run2.wig 0.025 0.5 21.7 13 306.0 2458212 2.3 7.1 -0.078 LB_culture_run2.wig 0.025 0.7 29.0 19 307.0 3270725 1.9 4.8 -0.160 growthout_control_24h_run2.wig 0.024 0.8 31.9 22 333.0 3492192 1.8 4.2 -0.082 intracellular_mutants_24h_run2.wig 0.021 0.3 15.0 6 285.0 1405508 3.0 11.3 0.080 extracellular_mutants_24h_run2.wig 0.011 0.1 4.7 3 159.0 236640 5.2 66.9 0.585 * example: Example method that calculates mean read-counts per gene. * transit example initial_mutants_run2.wig,LB_culture_run2.wig growthout_control_24h_run2.wig intracellular_mutants_24h_run2.wig extracellular_mutants_24h_run2.wig merged_genome.prot_table mean_read-counts_per_gene.txt #-->ERROR! #Orf Name Desc k n mean nzmean YWA314_00005 IS1329 transposase A 15 307 1.11 22.80 YWA314_00010 transposase B 7 243 0.30 10.57 YWA314_00015 hypothetical protein 0 103 0.00 0.00 YWA314_00020 phage protein 2 190 0.02 2.00 YWA314_00025 putative phage endopeptidase 1 418 0.05 20.00 * rankproduct: Rank product test for determining conditional essentiality. transit rankproduct LB_culture_run2.wig intracellular_mutants_24h_run2.wig merged_genome.prot_table rankproduct_out #-s 100 -n TTR -h -a -l #warnings.warn("\nOne or more of your .wig files does not include any empty sites (i.e. sites with zero read-counts). Proceeding as if data was Tn5 (all other sites assumed to be zero)!\n") #ERROR! #Tn5Gaps method: https://training.galaxyproject.org/training-material/topics/genome-annotation/tutorials/tnseq/tutorial.html#predict-the-essentiality-of-genes ** tn5gaps: It is based on a Gumbel analysis method Griffin et al. 2011 and adapted to Tn5 transposon specificity. The main difference comes from the fact that Tn5 transposon can insert everywhere, thus creating libraries with lower insertion rates. #transit tn5gaps initial_mutants_run2.wig merged_genome.prot_table initial_mutants_tn5gaps_out #-m 2 -r Sum -iN 5 -iC 5; for sample in initial_mutants LB_culture growthout_control_24h intracellular_mutants_24h extracellular_mutants_24h; do transit tn5gaps ${sample}_run2_normalized.wig merged_genome.prot_table ${sample}_tn5gaps_trimmed.dat -m 2 -r Sum -iN 5 -iC 5; done #grep "Essential" initial_mutants_tn5gaps_trimmed.dat | wc -l #298 #grep "Non-essential" initial_mutants_tn5gaps_trimmed.dat | wc -l #3834 ~/Tools/csv2xls-0.4/csv_to_xls.py initial_mutants_tn5gaps_trimmed.dat LB_culture_tn5gaps_trimmed.dat \ growthout_control_24h_tn5gaps_trimmed.dat intracellular_mutants_24h_tn5gaps_trimmed.dat extracellular_mutants_24h_tn5gaps_trimmed.dat -d$'\t' -o Tn5Gaps.xls; draw graphics to explain the r, ovr and lenovr based on the information below. #Orf Name Desc k n(length) r ovr lenovr pval padj call YWA314_00005 IS1329 transposase A 15 278 102 104 157 1.00000 1.00000 Non-essential YWA314_00010 transposase B 7 220 54 55 70 1.00000 1.00000 Non-essential YWA314_00015 hypothetical protein 0 94 94 96 502 0.29242 1.00000 Non-essential YWA314_00020 phage protein 2 173 113 114 502 0.29242 1.00000 Non-essential YWA314_00025 putative phage endopeptidase 1 379 315 317 439 0.81781 1.00000 Non-essential YWA314_17634 dnaA chromosomal replication initiation protein 1 1250 1137 1141 1287 0.00000 0.00000 Essential k: Number of Transposon Insertions Observed within the ORF. n: Total Number of TA dinucleotides within the ORF. r: Length of the Maximum Run of Non-Insertions observed. #TODO1_DEL: ovr: The number of nucleotides in the overlap with the longest run partially covering the gene. lenovr: The length of the above run with the largest overlap with the gene. pval: P-value calculated by the permutation test. padj: Adjusted p-value controlling for the FDR (Benjamini-Hochberg). call: Essentiality call for the gene. Depends on FDR corrected thresholds. Essential or Non-Essential. r (Run of Non-Insertions): This value represents the length of the longest continuous region within an ORF (open reading frame) where no transposon insertions are observed. Graphically, this could be shown as a long unbroken line or bar within a longer gene representation, highlighting the absence of marks (insertions). ovrovr (Overlap with Run): This is the number of nucleotides that overlap with the longest run, which might partially cover the gene. It is not the total length of the run, but how much of it overlaps with the gene. In a graphic, this could be illustrated by overlapping two segments: one for the gene and another for the run, with the overlapping part distinctly colored or shaded. lenovrlenovr (Length of Overlap with Run): This measures the full length of the run that has the largest overlap with the gene. Visually, this could be depicted as a separate longer line or bar that extends beyond the gene boundaries but is highlighted where it overlaps with the gene. * #WARNING: Since gumbel_out cannot be generated, the ttnfitness can be generated! * TTN-Fitness (TTNFitness method that calculates mean read-counts per gene): Typically with individual TnSeq datasets, Gumbel and HMM are the methods used for evaluating essentiality. - Gumbel distinguishes between ES (essential) from NE (non-essential). - HMM adds the GD (growth-defect; suppressed counts; mutant has reduced fitness) and GA (growth advantage; inflated counts; mutant has selective advantage) categories. - Quantifying the magnitude of the fitness defect is risky because the counts at individual TA sites can be noisy. Sometimes the counts at a TA site in a gene can span a wide range of very low to very high counts. The TTN-Fitness gives a more fine-grained analysis of the degree of fitness effect by taking into account the insertion preferences of the Himar1 transposon. - These insertion preferences are influenced by the nucleotide context of each TA site. The TTN-Fitness method uses a statistical model based on surrounding nucleotides to estimate the insertion bias of each site. Then, it corrects for this to compute an overall fitness level as a Fitness Ratio, where the ratio is 0 for ES genes, 1 for typical NE genes, between 0 and 1 for GD genes and above 1 for GA genes. transit ttnfitness <comma-separated .wig files> <annotation .prot_table> <genome .fna> <gumbel output file> <output1 file> <output2 file> transit ttnfitness initial_mutants_run2_normalized.wig,LB_culture_run2_normalized.wig,growthout_control_24h_run2_normalized.wig,intracellular_mutants_24h_run2_normalized.wig,extracellular_mutants_24h_run2_normalized.wig merged_genome.prot_table merged_genome.fasta gumbel_out ttnfitness_out1 ttnfitness_out2 transit ttnfitness initial_mutants_run2_normalized.wig,LB_culture_run2_normalized.wig,growthout_control_24h_run2_normalized.wig,intracellular_mutants_24h_run2_normalized.wig,extracellular_mutants_24h_run2_normalized.wig merged_genome.prot_table <genome .fna> <gumbel output file> <gene-wise output file> <ta-site wise output file> - gumbel output file:* The Gumbel method must be run first on the dataset.The output of the Gumbel method is provided as an input to this method. ES (essential by Gumbel) and EB (essential by Binomial) is calculated in the TTN-Fitness method via this files * Genetic Interactions: The genetic interactions (GI) method is a comparative analysis used used to determine genetic interactions. It is a Bayesian method that estimates the distribution of log fold-changes (logFC) in two strain backgrounds under different conditions, and identifies significantly large changes in enrichment (delta_logFC) to identify those genes that imply a genetic interaction. * Pathway Enrichment Analysis: Pathway Enrichment Analysis provides a method to identify enrichment of functionally-related genes among those that are conditionally essential (i.e. significantly more or less essential between two conditions). transit pathway_enrichment <resampling_file> <associations> <pathways> <output_file> [-M <FET|GSEA|GO>] [- ... ** corrplot (Correlation among TnSeq datasets, command line only): A useful tool when evaluating the quality of a collection of TnSeq datasets is to make a correlation plot of the mean insertion counts (averaged at the gene-level) among samples. #INCOMPLETE cut -f1-6 combined.wig > combined_.wig transit corrplot combined_.wig corrplot.png #INCOMPLETE cut -f1-6 combined_normalized.wig > combined_normalized_.wig transit corrplot combined_normalized_.wig corrplot_normalized.png transit corrplot anova_5samples_ref_LB_culture_out corrplot_anova.png -anova #[corrplot] Starting Corrplot correlations based on 74 genes [corrplot] Finished Corrplot ** heatmap (Heatmap among Conditions, command line only): The output of ANOVA or ZINB can be used to generate a heatmap that simultaneously clusters the significant genes and clusters the conditions, which is especially useful for shedding light on the relationships among the conditions apparent in the data. transit heatmap anova_5samples_ref_LB_culture_out heatmap.png -anova -qval 0.05 -low_mean_filter 3 #heatmap based on 74 genes transit heatmap anova_5samples_ref_LB_culture_out heatmap.png -anova -qval 0.1 -low_mean_filter 3 #-- convert gbk to prot_table -- python3 ~/Scripts/gbk_to_prottable.py merged_genome.gbk merged_genome.prot_table #transit export #https://transit.readthedocs.io/en/latest/transit_normalization_tutorial.html #-1- transit export combined_wig <comma-separated .wig files> <annotation .prot_table> <output file> -n TTR #RUN transit export combined_wig initial_mutants_run2.wig,LB_culture_run2.wig,growthout_control_24h_run2.wig,intracellular_mutants_24h_run2.wig,extracellular_mutants_24h_run2.wig merged_genome.prot_table combined.wig -n nonorm transit export combined_wig initial_mutants_run2.wig,LB_culture_run2.wig,growthout_control_24h_run2.wig,intracellular_mutants_24h_run2.wig,extracellular_mutants_24h_run2.wig merged_genome.prot_table combined_normalized.wig -n TTR #-2- transit export igv initial_mutants_run2.wig,LB_culture_run2.wig,growthout_control_24h_run2.wig,intracellular_mutants_24h_run2.wig,extracellular_mutants_24h_run2.wig merged_genome.prot_table combined_normalized.igv -n TTR #TODO: replace merged_genome to WA314! #DEBUG: how to run it? #-3- transit export mean_counts -c combined.wig combined.mean_counts # note: append -c if inputing a combined_wig file # -- detect essential genes (DnaA is essential, which is why there are no insertion counts in the first few TA sites) #https://transit.readthedocs.io/en/v3.2.5/method_ttnfitness.html #对于 transit gumbel 分析,通常期望输入的是已经规范化(normalized)的 .wig 文件。规范化的数据可以减少由于不同实验条件或测序深度导致的偏差,使得后续的统计分析更加可靠 #-- Normalization -- #https://transit.readthedocs.io/en/latest/method_normalization.html for sample in initial_mutants LB_culture growthout_control_24h intracellular_mutants_24h extracellular_mutants_24h; do transit normalize ${sample}_run2.wig ${sample}_run2_normalized.wig -n TTR #betageom done #https://transit.readthedocs.io/en/latest/method_tnseq_stats.html #pre-normalization for sample in initial_mutants LB_culture growthout_control_24h intracellular_mutants_24h extracellular_mutants_24h; do transit tnseq_stats ${sample}_run2.wig done #post-normalization for sample in initial_mutants LB_culture growthout_control_24h intracellular_mutants_24h extracellular_mutants_24h; do transit tnseq_stats ${sample}_run2_normalized.wig done -
Reports
#1. For gene_based reports: example (mean_read-counts_per_gene.txt) + ANOVA (anova_5samples_ref_LB_culture_out) #TAs: Number of TA sites in Gene #mean: Average read-count, including empty sites. #nzmean: Average read-count, excluding empty sites. Orf: Gene ID Name: Name of the gene. Desc Gene description. k: Number of Transposon Insertions Observed within the ORF. n: Total Number of TA sites within the ORF Normalized_initial_mutants: Mean read counts for the condition initial_mutants (normalized with TTR) Normalized_LB_culture: Mean read counts for the condition LB_culture (normalized with TTR) Normalized_growthout_control_24h: Mean read counts for the condition growthout_control_24h (normalized with TTR) Normalized_intracellular_mutants_24h: Mean read counts for the condition intracellular_mutants_24h (normalized with TTR) Normalized_extracellular_mutants_24h: Mean read counts for the condition extracellular_mutants_24h (normalized with TTR) Orf Name Desc k n mean nzmean Rv Gene TAs Normalized_initial_mutants Normalized_LB_culture Normalized_growthout_control_24h Normalized_intracellular_mutants_24h Normalized_extracellular_mutants_24h #check both f1 are the same; cut -f4-8 anova_5samples_ref_LB_culture_out > f4_8; paste mean_read-counts_per_gene.txt f4_8 > overview_gene_based.txt #delete the columns mean and nzmean. #2. For essentiall gene report: transit tn5gaps TODO: delete the headers; DEL ovr; merge tn5gaps also the gene-based tables, add columns r, ovr, lenovr, pval, padj, call to the gene-based table. #Tn5Gaps method: https://training.galaxyproject.org/training-material/topics/genome-annotation/tutorials/tnseq/tutorial.html#predict-the-essentiality-of-genes #dinucleotides ORF Gene ID. Name Name of the gene. Desc Gene description. k Number of Transposon Insertions Observed within the ORF. n Total Number of TA sites within the ORF. r Length of the Maximum Run of Non-Insertions observed. pval P-value calculated by the permutation test. padj Adjusted p-value controlling for the FDR (Benjamini-Hochberg). call Essentiality call for the gene. Depends on FDR corrected thresholds. Essential or Non-Essential. #3. Only choose ANOVA for DEG reports, since it can be drawn + are consistent with the gene-based view! # delete MSR and MSE+alpha. Orf: Gene ID Name: Name of the gene TAs: Number of TA sites in the gene Means…: Mean read counts for each condition LFCs…: Log-fold changes of counts in each condition vs. the mean across all conditions p-value: P-value calculated by the ANOVA test p-adj: Adjusted p-value controlling for the FDR (False Discovery Rate, Benjamini-Hochberg method) status: Debug information #4. 2 plots (Temporarily do not send, since the based on 74 genes, we have only 30+ significant genes between LB_culture and intracellular_mutants_24h!) corrplot: A useful tool when evaluating the quality of a collection of TnSeq datasets is to make a correlation plot of the mean insertion counts (averaged at the gene-level) among samples. heatmap: The output of ANOVA can be used to generate a heatmap that simultaneously clusters the significant genes and clusters the conditions, which is especially useful for shedding light on the relationships among the conditions apparent in the data. #5. (Temporarily do not send, since for previous wig files can also beed used!) five wig-files and merged_genome.gb and merged_genome.fa #resampling #Orf Name Desc Sites Mean Ctrl Mean Exp log2FC Sum Ctrl Sum Exp Delta Mean p-value Adj. p-value YWA314_00463 chaperone protein DnaJ 1129 38.5 1.3 -4.13 43427.8 1413.92 -37.2 0 0 #utest #Orf Name Desc Sites Mean Ctrl Mean Exp log2FC U-Statistic p-value Adj. p-value YWA314_00463 chaperone protein DnaJ 1129 1696.7 339.9 -2.32 635 0 0 #ANOVA #Rv Gene TAs Mean_LB_culture Mean_intracellular_mutants_24h LFC_LB_culture LFC_intracellular_mutants_24h MSR MSE+alpha Fstat Pval Padj status YWA314_05189 2506 106.58 0 0 -4.48 14054884.5521 338643.330313 41.503503 0 0.000001 YWA314_00518 carB 3232 194.02 31.97 0 -2.428 42162662.154189 1121416.346908 37.597688 0 0.000002 YWA314_16630 2146 34.88 0 0 -2.996 1261390.068193 36940.341092 34.146682 0 0.000008 YWA314_00463 1129 49.05 1.87 0 -2.976 1051922.250591 110643.16916 9.50734 0.002071 0.213913 YWA314_00463 1129 58.38 49.05 19.61 1.87 22.56 0.230 0.000 -1.135 -2.976 -0.972 489073.319278 169569.370262 2.884208 0.021237 0.669899 -
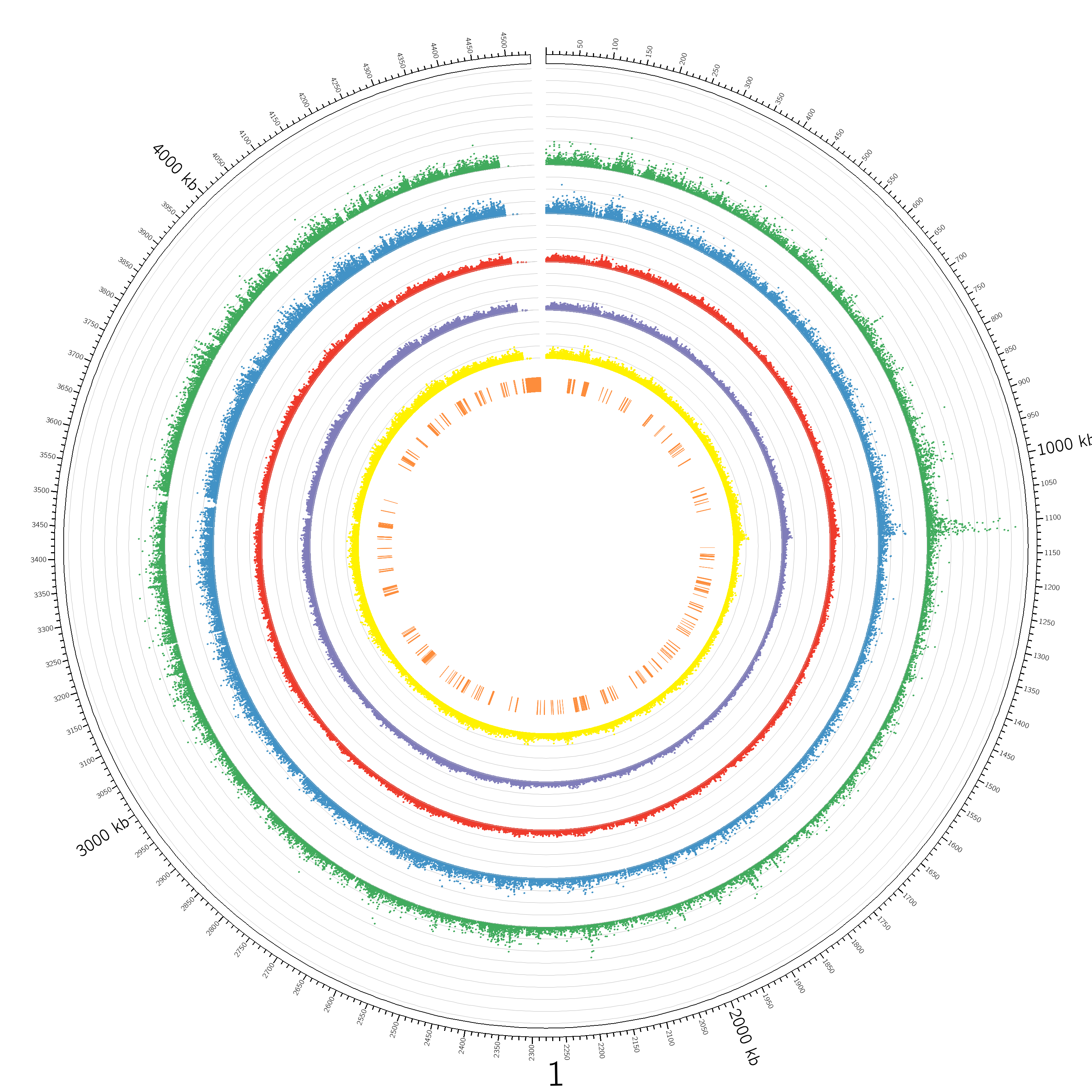
plot Figure 1. Overview of the Yersinia enterocolitica subsp. enterocolitica WA-314 Transposon Mutant Library This figure illustrates the distribution of transposon insertion sites across the genome. The outermost black circle represents the WA-314 genome in kilobase pairs (Kbp). The middle circles, which feature scatter points, indicate the normalized number of sequencing reads at each unique transposon insertion site, with each axis line representing an increment of 50,000 in data values. The circles are color-coded to represent different conditions: green for extracellular mutants, blue for intracellular mutants, red for growthout control, purple for LB culture, and yellow for initial mutants. The innermost orange circle highlights the locations of genes identified as essential.
#circos -conf circos.conf <<include /etc/circos/colors_fonts_patterns.conf>> karyotype = circos_data/karyotype.microbe.txt chromosomes_units = 10000 chromosomes_display_default = yes <ideogram> show = yes <spacing> default = 0.01r break = 0.5r #8r # Adds a gap between the first and last position of the single chromosome </spacing> radius = 0.9r thickness = 25p fill = no stroke_thickness = 2 stroke_color = black show_bands = yes fill_bands = yes band_transparency = 0 show_label = yes label_font = default label_radius = 1.05r label_size = 75 label_parallel = yes orientation = 100 # Rotate the plot by 90 degrees </ideogram> #<<include ticks.conf>> show_ticks = yes show_tick_labels = yes show_grid = no grid_start = dims(ideogram,radius_inner)-0.5r grid_end = dims(ideogram,radius_inner) <ticks> skip_first_label = yes skip_last_label = no radius = dims(ideogram,radius_outer) tick_separation = 2p min_label_distance_to_edge = 0p label_separation = 5p label_offset = 5p label_size = 8p multiplier = 0.001 color = black thickness = 3p size = 20p <tick> size = 10p spacing = 1u color = black show_label = no label_size = 12p format = %.2f grid = no grid_color = lblue grid_thickness = 1p </tick> <tick> size = 15p spacing = 5u color = black show_label = yes label_size = 16p format = %s grid = yes grid_color = lgrey grid_thickness = 1p </tick> <tick> size = 18p spacing = 10u color = black show_label = yes label_size = 16p format = %s grid = yes grid_color = grey grid_thickness = 1p </tick> <tick> spacing = 100u color = black show_label = yes suffix = " kb" label_size = 36p format = %s grid = yes grid_color = dgrey grid_thickness = 1p </tick> </ticks> <image> <<include /etc/circos/image.conf>> </image> <colors> <<include /etc/circos/colors.conf>> </colors> <fonts> <<include /etc/circos/fonts.conf>> </fonts> # -- Scatter plot 1 -- <plots> <plot> type = scatter file = circos_data/extracellular_mutants.txt r1 = 0.99r r0 = 0.79r #python3 identify_min_max.py circos_data/initial_mutants.txt min = 0 max = 400000 glyph = circle glyph_size = 5 color = dgreen <axes> <axis> spacing = 50000 color = lgrey </axis> </axes> #<axes> #<axis> #spacing = 0.1r #color = lgrey #<ticks> #<tick> #spacing = 0.1 #size = 10p #thickness = 2p #color = lgrey #show_label = yes #label_size = 20p #label_offset = 5p #format = %0.1f #</tick> #</ticks> #</axis> #</axes> #<rules> #<rule> #condition = var(value) > 10000 #stroke_color = dred #fill_color = red #glyph = rectangle #glyph_size = 2 #</rule> #</rules> </plot> # -- Scatter plot 2 -- <plot> type = scatter file = circos_data/intracellular_mutants.txt r1 = 0.79r r0 = 0.69r min = 0 max = 200000 glyph = circle glyph_size = 5 color = dblue <axes> <axis> spacing = 50000 color = lgrey </axis> </axes> </plot> # -- Scatter plot 3 -- <plot> type = scatter file = circos_data/growthout_control.txt r1 = 0.69r r0 = 0.59r min = 0 max = 200000 glyph = circle glyph_size = 5 color = dred <axes> <axis> spacing = 50000 color = lgrey </axis> </axes> </plot> # -- Scatter plot 4 -- <plot> type = scatter file = circos_data/LB_culture.txt r1 = 0.59r r0 = 0.49r min = 0 max = 200000 glyph = circle glyph_size = 5 color = dpurple <axes> <axis> spacing = 50000 color = lgrey </axis> </axes> </plot> # -- Scatter plot 5 -- <plot> type = scatter file = circos_data/initial_mutants.txt r1 = 0.49r r0 = 0.39r min = 0 max = 200000 glyph = circle glyph_size = 5 color = dyellow <axes> <axis> spacing = 50000 color = lgrey </axis> </axes> </plot> # Gene Locations <plot> type = heatmap file = circos_data/merged_genome.txt r1 = 0.35r r0 = 0.32r color = orange </plot> #grep "Essential" tn5_gap_inituial_mutant.csv > essential_genes.txt #cut -f1 -d$'\t' essential_genes.txt > f1 #vim merged_genome.prot_table #" merged_genome.prot_table >> merged_genome.prot_table_essential \ngrep " #python3 generate_gene_locations.py #replace "\n\t" with "\nchr\t" </plots> <<include /etc/circos/housekeeping.conf>>
点赞本文的读者
还没有人对此文章表态
本文有评论
没有评论
看文章,发评论,不要沉默
最受欢迎文章
- Motif Discovery in Biological Sequences: A Comparison of MEME and HOMER
- Why Do Significant Gene Lists Change After Adding Additional Conditions in Differential Gene Expression Analysis?
- Calling peaks using findPeaks of HOMER
- PiCRUST2 Pipeline for Functional Prediction and Pathway Analysis in Metagenomics
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- pheatmap vs heatmap.2
- Should the inputs for GSVA be normalized or raw?
- Setup conda environments
- Kraken2 Installation and Usage Guide
- File format for single channel analysis of Agilent microarray data with Limma?
最新文章
- Setup the environment for lumicks-pylake and C_Trap-Multimer-photontrack.ipynb
- 🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
- MCV病毒中的LT与sT蛋白功能
- Analysis of the RNA binding protein (RBP) motifs for RNA-Seq and miRNAs (v3, simplied)
最多评论文章
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- The top 10 genes
- Retrieving KEGG Genes Using Bioservices in Python
推荐相似文章
Setup the environment for lumicks-pylake and C_Trap-Multimer-photontrack.ipynb
🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
Analysis of the RNA binding protein (RBP) motifs for RNA-Seq and miRNAs (v3, simplied)