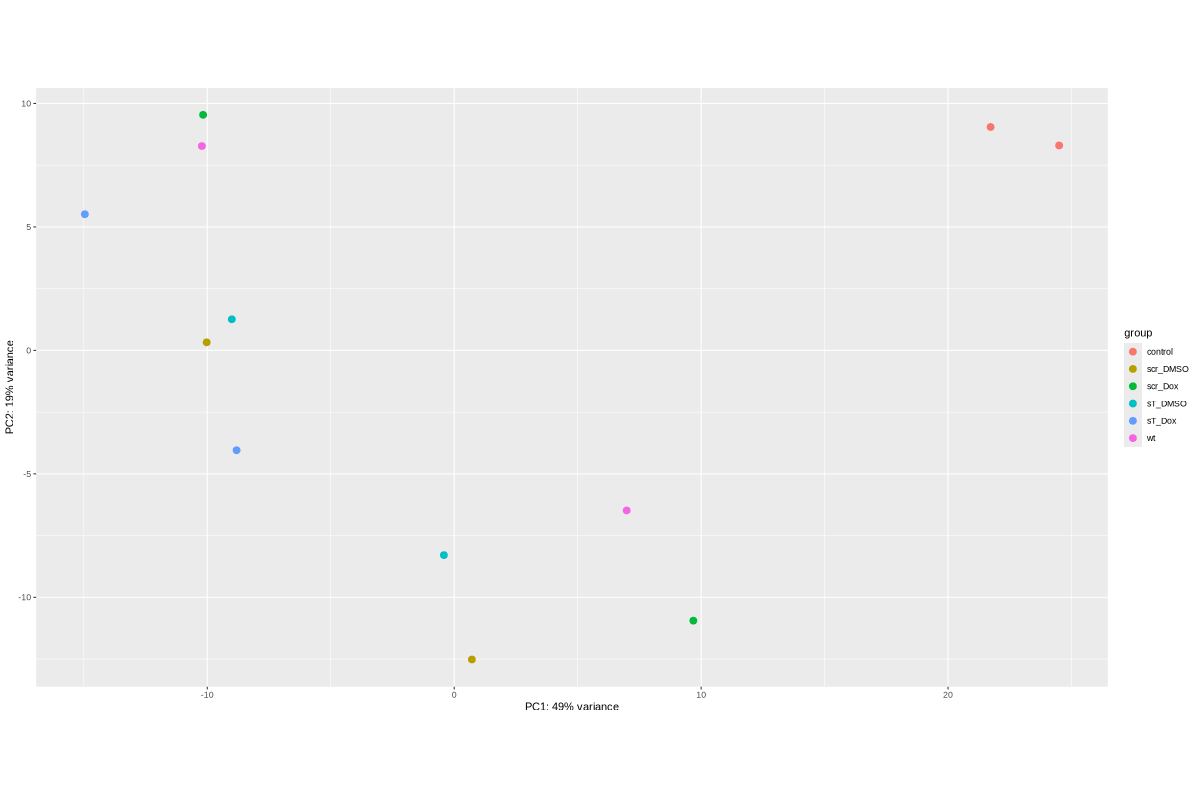
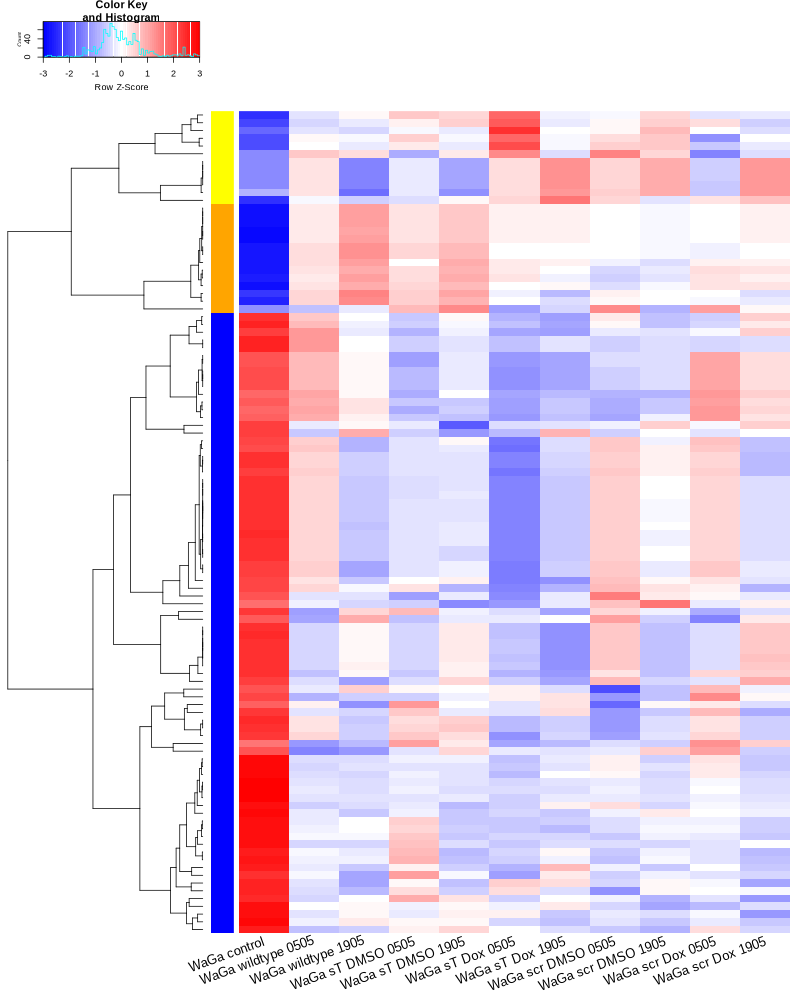
Draw plots for snRNA generated by COMPSRA
gene_x 0 like s 676 view s
Tags: pipeline
-
Generate the following files according to STEPS 1-4 from http://xgenes.com/article/article-content/239/small-rna-sequencing-processing-in-the-example-of-smallrna-7/, http://xgenes.com/article/article-content/232/small-rna-sequencing-processing-in-the-example-of-smallrna-7/, and http://xgenes.com/article/article-content/156/small-rna-processing/. For COMPSRA_MERGE_0_miRNA.txt, we also need STEP 5 to add the read numbers of MCPyV-M1.
COMPSRA_MERGE_0_miRNA.txt COMPSRA_MERGE_0_piRNA.txt COMPSRA_MERGE_0_snRNA.txt * COMPSRA_MERGE_0_tRNA.txt COMPSRA_MERGE_0_snoRNA.txt COMPSRA_MERGE_0_circRNA.txt -
Input files for snRNA are two files: COMPSRA_MERGE_0_snRNA.txt and ids
-
COMPSRA_MERGE_0_snRNA.txt
#The former are more precise due to the reads from virus will be mapped on the virus-genome diff ./our_out_on_hg38+JN707599/COMPSRA_MERGE_0_snRNA.txt ./our_out_on_hg38/COMPSRA_MERGE_0_snRNA.txt cp ../our_out_on_hg38+JN707599/COMPSRA_MERGE_0_snRNA.txt .
-
prepare the file ids
#see Option4: manully defining
-
-
Draw plots with R using DESeq2
#BiocManager::install("AnnotationDbi") #BiocManager::install("clusterProfiler") #BiocManager::install(c("ReactomePA","org.Hs.eg.db")) #BiocManager::install("limma") library("AnnotationDbi") library("clusterProfiler") library("ReactomePA") library("org.Hs.eg.db") library(DESeq2) library(gplots) library(limma) # Check the current library paths .libPaths() #setwd("/home/jhuang/DATA/Data_Ute/Data_Ute_smallRNA_7/our_out_on_hg38+JN707599_2024_corrected/") d.raw<- read.delim2("COMPSRA_MERGE_0_snRNA.txt",sep="\t", header=TRUE, row.names=1) d.raw$X <- NULL d.raw[] <- lapply(d.raw, as.numeric) EV_or_parental = as.factor(c("EV","EV", "EV","EV", "EV","EV", "EV","EV", "EV","EV", "parental","parental")) donor = as.factor(c("0505","1905", "0505","1905", "0505","1905", "0505","1905", "0505","1905", "0505","1905")) replicates = as.factor(c("sT_DMSO","sT_DMSO", "sT_Dox","sT_Dox", "scr_DMSO","scr_DMSO", "scr_Dox","scr_Dox", "wt","wt", "control","control")) ids = as.factor(c("0505_WaGa_sT_DMSO","1905_WaGa_sT_DMSO","0505_WaGa_sT_Dox","1905_WaGa_sT_Dox","0505_WaGa_scr_DMSO","1905_WaGa_scr_DMSO","0505_WaGa_scr_Dox","1905_WaGa_scr_Dox","0505_WaGa_wt","1905_WaGa_wt","control_MKL1","control_WaGa")) cData = data.frame(row.names=colnames(d.raw), replicates=replicates, ids=ids, donor=donor, EV_or_parental=EV_or_parental) dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~replicates+donor) rld <- rlogTransformation(dds) # -- before pca -- png("pca.png", 1200, 800) plotPCA(rld, intgroup=c("replicates")) #plotPCA(rld, intgroup = c("replicates", "batch")) #plotPCA(rld, intgroup = c("replicates", "ids")) #plotPCA(rld, "batch") dev.off() png("pca2.png", 1200, 800) plotPCA(rld, intgroup=c("donor")) dev.off() #### STEP2: DEGs #### #convert bam to bigwig using deepTools by feeding inverse of DESeq’s size Factor sizeFactors(dds) #NULL dds <- estimateSizeFactors(dds) sizeFactors(dds) normalized_counts <- counts(dds, normalized=TRUE) write.table(normalized_counts, file="normalized_counts.txt", sep="\t", quote=F, col.names=NA) #---- * to untreated ---- dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~EV_or_parental+donor) dds$EV_or_parental <- relevel(dds$EV_or_parental, "parental") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("EV_vs_parental") for (i in clist) { contrast = paste("EV_or_parental", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] #https://bioconductor.org/packages/release/bioc/vignettes/DESeq2/inst/doc/DESeq2.html#why-are-some-p-values-set-to-na res$padj <- ifelse(is.na(res$padj), 1, res$padj) res_df <- as.data.frame(res) write.csv(as.data.frame(res_df[order(res_df$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(res_df, padj<=0.1 & log2FoldChange>=2) down <- subset(res_df, padj<=0.1 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) } #~/Tools/csv2xls-0.4/csv_to_xls.py EV_vs_parental-all.txt EV_vs_parental-up.txt EV_vs_parental-down.txt -d$',' -o EV_vs_parental.xls; dds<-DESeqDataSetFromMatrix(countData=d.raw, colData=cData, design=~replicates+donor) dds$replicates <- relevel(dds$replicates, "sT_DMSO") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("sT_Dox_vs_sT_DMSO") dds$replicates <- relevel(dds$replicates, "scr_Dox") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("sT_Dox_vs_scr_Dox") dds$replicates <- relevel(dds$replicates, "scr_DMSO") dds = DESeq(dds, betaPrior=FALSE) resultsNames(dds) clist <- c("scr_Dox_vs_scr_DMSO", "sT_DMSO_vs_scr_DMSO") for (i in clist) { contrast = paste("replicates", i, sep="_") res = results(dds, name=contrast) res <- res[!is.na(res$log2FoldChange),] #https://bioconductor.org/packages/release/bioc/vignettes/DESeq2/inst/doc/DESeq2.html#why-are-some-p-values-set-to-na res$padj <- ifelse(is.na(res$padj), 1, res$padj) res_df <- as.data.frame(res) write.csv(as.data.frame(res_df[order(res_df$pvalue),]), file = paste(i, "all.txt", sep="-")) up <- subset(res_df, padj<=0.1 & log2FoldChange>=2) down <- subset(res_df, padj<=0.1 & log2FoldChange<=-2) write.csv(as.data.frame(up[order(up$log2FoldChange,decreasing=TRUE),]), file = paste(i, "up.txt", sep="-")) write.csv(as.data.frame(down[order(abs(down$log2FoldChange),decreasing=TRUE),]), file = paste(i, "down.txt", sep="-")) } ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_Dox_vs_sT_DMSO-all.txt \ sT_Dox_vs_sT_DMSO-up.txt \ sT_Dox_vs_sT_DMSO-down.txt \ -d$',' -o sT_Dox_vs_sT_DMSO.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_Dox_vs_scr_Dox-all.txt \ sT_Dox_vs_scr_Dox-up.txt \ sT_Dox_vs_scr_Dox-down.txt \ -d$',' -o sT_Dox_vs_scr_Dox.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ scr_Dox_vs_scr_DMSO-all.txt \ scr_Dox_vs_scr_DMSO-up.txt \ scr_Dox_vs_scr_DMSO-down.txt \ -d$',' -o scr_Dox_vs_scr_DMSO.xls; ~/Tools/csv2xls-0.4/csv_to_xls.py \ sT_DMSO_vs_scr_DMSO-all.txt \ sT_DMSO_vs_scr_DMSO-up.txt \ sT_DMSO_vs_scr_DMSO-down.txt \ -d$',' -o sT_DMSO_vs_scr_DMSO.xls; ##### STEP3: prepare all_genes ##### rld <- rlogTransformation(dds) mat <- assay(rld) mm <- model.matrix(~replicates, colData(rld)) mat <- limma::removeBatchEffect(mat, batch=rld$donor, design=mm) assay(rld) <- mat RNASeq.NoCellLine <- assay(rld) # reorder the columns colnames(RNASeq.NoCellLine) = c("0505 WaGa sT DMSO","1905 WaGa sT DMSO","0505 WaGa sT Dox","1905 WaGa sT Dox","0505 WaGa scr DMSO","1905 WaGa scr DMSO","0505 WaGa scr Dox","1905 WaGa scr Dox","0505 WaGa wt","1905 WaGa wt","control MKL1","control WaGa") col.order <-c("control MKL1", "control WaGa","0505 WaGa wt","1905 WaGa wt","0505 WaGa sT DMSO","1905 WaGa sT DMSO","0505 WaGa sT Dox","1905 WaGa sT Dox","0505 WaGa scr DMSO","1905 WaGa scr DMSO","0505 WaGa scr Dox","1905 WaGa scr Dox") RNASeq.NoCellLine <- RNASeq.NoCellLine[,col.order] #Option4: manully defining #for i in EV_vs_parental sT_Dox_vs_sT_DMSO sT_Dox_vs_scr_Dox scr_Dox_vs_scr_DMSO sT_DMSO_vs_scr_DMSO; do echo "cut -d',' -f1-1 ${i}-up.txt > ${i}-up.id"; echo "cut -d',' -f1-1 ${i}-down.txt > ${i}-down.id"; done #cat *.id | sort -u > ids ##add Gene_Id in the first line, delete the "" GOI <- read.csv("ids")$Gene_Id datamat = RNASeq.NoCellLine[GOI, ] ##### STEP4: clustering the genes and draw heatmap ##### datamat <- datamat[,-1] #delete the sample "control MKL1" colnames(datamat)[1] <- "WaGa control" #rename the isolate names according to the style of RNA-seq as follows? colnames(datamat)[2] <- "WaGa wildtype 0505" colnames(datamat)[3] <- "WaGa wildtype 1905" colnames(datamat)[4] <- "WaGa sT DMSO 0505" colnames(datamat)[5] <- "WaGa sT DMSO 1905" colnames(datamat)[6] <- "WaGa sT Dox 0505" colnames(datamat)[7] <- "WaGa sT Dox 1905" colnames(datamat)[8] <- "WaGa scr DMSO 0505" colnames(datamat)[9] <- "WaGa scr DMSO 1905" colnames(datamat)[10] <- "WaGa scr Dox 0505" colnames(datamat)[11] <- "WaGa scr Dox 1905" write.csv(datamat, file ="gene_expression_keeping_replicates.txt") #"ward.D"’, ‘"ward.D2"’,‘"single"’, ‘"complete"’, ‘"average"’ (= UPGMA), ‘"mcquitty"’(= WPGMA), ‘"median"’ (= WPGMC) or ‘"centroid"’ (= UPGMC) hr <- hclust(as.dist(1-cor(t(datamat), method="pearson")), method="complete") hc <- hclust(as.dist(1-cor(datamat, method="spearman")), method="complete") mycl = cutree(hr, h=max(hr$height)/1.5) mycol = c("YELLOW", "BLUE", "ORANGE", "CYAN", "GREEN", "MAGENTA", "GREY", "LIGHTCYAN", "RED", "PINK", "DARKORANGE", "MAROON", "LIGHTGREEN", "DARKBLUE", "DARKRED", "LIGHTBLUE", "DARKCYAN", "DARKGREEN", "DARKMAGENTA"); mycol = mycol[as.vector(mycl)] png("snRNA_heatmap_keeping_replicates.png", width=800, height=1000) #svg("DEGs_heatmap_keeping_replicates.svg", width=6, height=8) heatmap.2(as.matrix(datamat), Rowv=as.dendrogram(hr), Colv=NA, dendrogram='row', labRow="", scale='row', trace='none', col=bluered(75), RowSideColors=mycol, srtCol=20, lhei=c(1,8), #cexRow=1.2, # Increase row label font size cexCol=1.7, # Increase column label font size margin=c(7,1) ) dev.off() #### cluster members ##### write.csv(names(subset(mycl, mycl == '1')),file='YELLOW.txt') write.csv(names(subset(mycl, mycl == '2')),file='BLUE.txt') write.csv(names(subset(mycl, mycl == '3')),file='ORANGE.txt') #~/Tools/csv2xls-0.4/csv_to_xls.py gene_expression_keeping_replicates.txt YELLOW.txt ORANGE.txt BLUE.txt -d',' -o snRNA_heatmap_keeping_replicates.xls mv snRNA_heatmap_keeping_replicates.png snRNA_heatmap.png mv snRNA_heatmap_keeping_replicates.xls snRNA_heatmap.xls mv pca.png snRNA_pca.png mv EV_vs_parental.xls snRNA_EV_vs_parental.xls mv sT_DMSO_vs_scr_DMSO.xls snRNA_sT_DMSO_vs_scr_DMSO.xls mv sT_Dox_vs_scr_Dox.xls snRNA_sT_Dox_vs_scr_Dox.xls mv sT_Dox_vs_sT_DMSO.xls snRNA_sT_Dox_vs_sT_DMSO.xls mv scr_Dox_vs_scr_DMSO.xls snRNA_scr_Dox_vs_scr_DMSO.xls # --> SENDING snRNA_*.png, snRNA_EV_vs_parental.xls, and snRNA_heatmap.xls # ---- NOT WORKING WELL ---- # merging replicates datamat <- cbind(datamat, "WaGa wildtype" = rowMeans(datamat[, 2:3])) datamat <- cbind(datamat, "WaGa sT DMSO" = rowMeans(datamat[, 4:5])) datamat <- cbind(datamat, "WaGa sT Dox" = rowMeans(datamat[, 6:7])) datamat <- cbind(datamat, "WaGa scr DMSO" = rowMeans(datamat[, 8:9])) datamat <- cbind(datamat, "WaGa scr Dox" = rowMeans(datamat[, 10:11])) datamat <- datamat[,c(-2:-11)] write.csv(datamat, file ="gene_expression_merging_replicates.txt") # Ensure 'mycl' is calculated properly. mycl <- cutree(hr, h=max(hr$height)/2.9) # mycol = c("YELLOW", "BLUE", "ORANGE", "CYAN", "GREEN", "MAGENTA", "GREY", "LIGHTCYAN", "RED", "PINK", "DARKORANGE", "MAROON", "LIGHTGREEN", "DARKBLUE", "DARKRED", "LIGHTBLUE", "DARKCYAN", "DARKGREEN", "DARKMAGENTA"); # Now map your clusters to colors, making sure that there's one color for each row: actualColors <- mycol[mycl] # Assign colors based on cluster assignment # Then use these 'actualColors' in your heatmap: png("snRNA_heatmap_merging_replicates.png", width=800, height=1000) heatmap.2(as.matrix(datamat), Rowv=as.dendrogram(hr), Colv=NA, dendrogram='row', labRow="", scale='row', trace='none', col=bluered(75), RowSideColors=actualColors, # Update this part srtCol=20, lhei=c(1,8), cexCol=1.7, # Increase column label font size margin=c(7,1) ) dev.off() #### cluster members ##### write.csv(names(subset(mycl, mycl == '1')),file='YELLOW.txt') write.csv(names(subset(mycl, mycl == '2')),file='BLUE.txt') write.csv(names(subset(mycl, mycl == '3')),file='ORANGE.txt') write.csv(names(subset(mycl, mycl == '4')),file='CYAN.txt') write.csv(names(subset(mycl, mycl == '5')),file='GREEN.txt') write.csv(names(subset(mycl, mycl == '6')),file='MAGENTA.txt') write.csv(names(subset(mycl, mycl == '7')),file='GREY.txt') write.csv(names(subset(mycl, mycl == '8')),file='LIGHTCYAN.txt') #write.csv(names(subset(mycl, mycl == '9')),file='RED.txt') #~/Tools/csv2xls-0.4/csv_to_xls.py gene_expression_merging_replicates.txt YELLOW.txt BLUE.txt ORANGE.txt CYAN.txt MAGENTA.txt GREEN.txt LIGHTCYAN.txt GREY.txt -d',' -o snRNA_heatmap_merging_replicates.xls
点赞本文的读者
还没有人对此文章表态
本文有评论
没有评论
看文章,发评论,不要沉默
最受欢迎文章
- Motif Discovery in Biological Sequences: A Comparison of MEME and HOMER
- Why Do Significant Gene Lists Change After Adding Additional Conditions in Differential Gene Expression Analysis?
- Calling peaks using findPeaks of HOMER
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- PiCRUST2 Pipeline for Functional Prediction and Pathway Analysis in Metagenomics
- pheatmap vs heatmap.2
- Should the inputs for GSVA be normalized or raw?
- Setup conda environments
- Kraken2 Installation and Usage Guide
- File format for single channel analysis of Agilent microarray data with Limma?
最新文章
- 🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
- MCV病毒中的LT与sT蛋白功能
- Analysis of the RNA binding protein (RBP) motifs for RNA-Seq and miRNAs (v3, simplied)
- Somatic Variation Detection
最多评论文章
- Updating Human Gene Identifiers using Ensembl BioMart: A Step-by-Step Guide
- The top 10 genes
- Retrieving KEGG Genes Using Bioservices in Python
推荐相似文章
🧬 Cadmium Resistance Gene Analysis in Staphylococcus epidermidis HD46
Analysis of the RNA binding protein (RBP) motifs for RNA-Seq and miRNAs (v3, simplied)